Machine learning algorithms are applied in molecular modelling, making it possible to develop new treatments ‘in silico’.
Robotics and digital imaging fostered the development of “minimally invasive” techniques. The convergence of intensive computing and artificial intelligence (AI) is now speeding up basic and clinical medical research, and is fostering the development of healthcare that is both personalised and predictive. As for blockchain, it offers a framework that helps address the challenges brought about by the explosion of health data in terms in terms of security, interoperability, regulatory compliance, and patient consent management.
Robot-assisted surgery
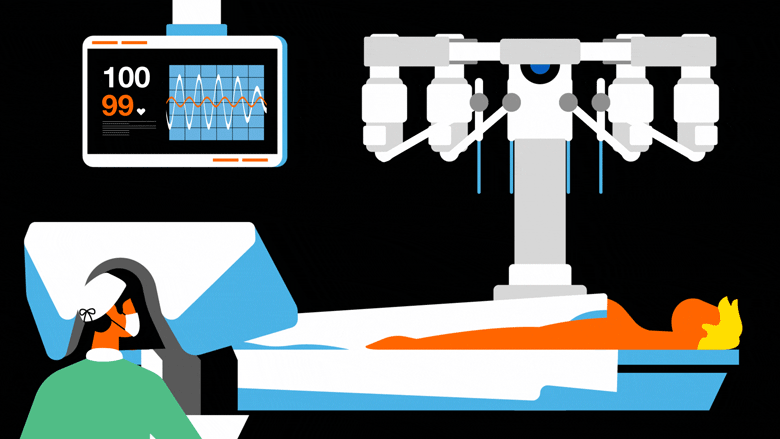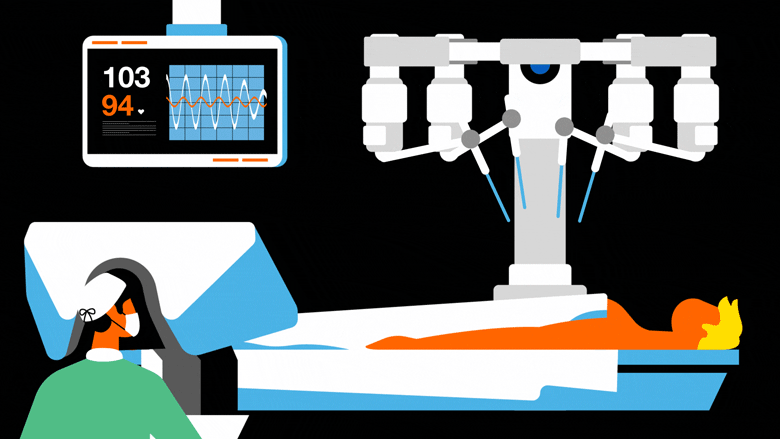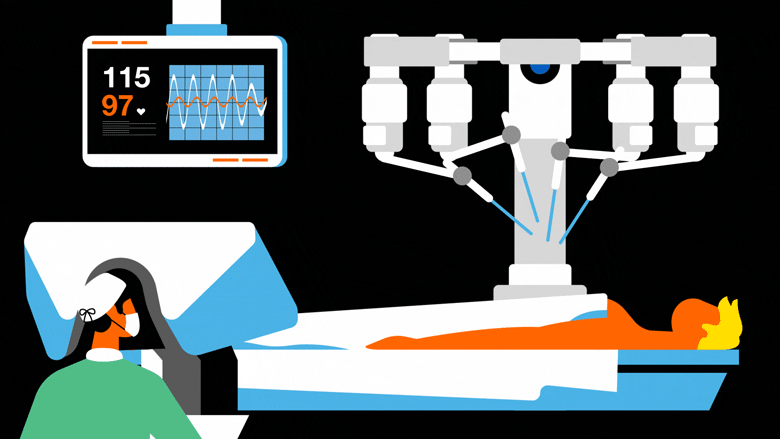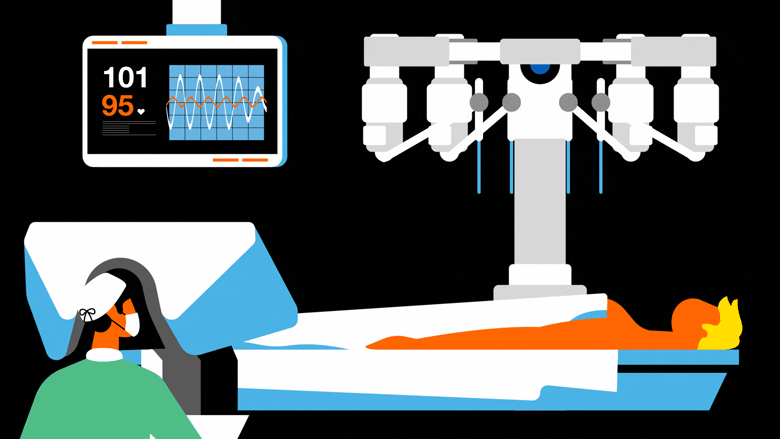
An integral part of computer-assisted surgery – a set of digital tools aimed at helping the surgeon in the preparation and carrying out of an operation –, robots first entered the operating theatre in the early 2000s.
Their use aims to improve the precision of the surgical gesture and, coupled with digital imaging, has fostered the development of so-called “minimally invasive” techniques that help to limit the traumas suffered by patients.
Some robots are remotely-operated, meaning they perform surgical gestures with the help of articulated arms and an array of tools, but are still controlled at a distance by the surgeon.
The most well-known example is the Da Vinci robot, developed by American firm Intuitive Surgical, used in particular during prostate surgery.
Da Vinci is made up of two parts. The first, which is situated above the patient, is equipped with four articulated arms, one of which holds an endoscope camera while the others manipulate the surgical instruments. The second part comprises a seat in which the surgeon is installed, two screens that relay the 3D images in real time, and two levers to control the instruments.
There are also autonomous robots that, once programmed, can perform interventions alone, such as the Cyberknife radiosurgery robot sold by Accuray, which enables treatment of tumours by delivering powerful and highly accurate radiation beams.
Supercomputers are accelerating medical research

In January 2020, IDRIS, the Institute for Development and Resources in Intensive Scientific Computing of the French national centre for scientific research, announced the acquisition of supercomputer Jean Zay, one of the most powerful in Europe. With a computing power of 16 petaflops (ten times more than the previous IDRIS supercomputer), Jean Zay is in part dedicated to AI, whose applications cover medical research.
Machine learning algorithms are being applied in molecular modelling, making it now possible to develop new treatments ‘in silico’ (that is through digital simulations).
However, training and running these algorithms requires high computing power. Supercomputers are bringing together intensive computing and AI to provide researchers with the power they need to simulate and analyse complex biological phenomena.
Currently, the computing power of Jean Zay is being put to use to study the molecular structure of Covid-19. The Sorbonne university theoretical chemistry laboratory, for example, is looking at the Spike protein, which enables the virus to interact with human cells.
“Failing destroying the pathogen, deactivating this protein would prevent the virus from penetrating and infecting host cells”, explains its director, Jean-Philip Piquemal.
However, the size and complexity of this protein make the calculations and models more cumbersome. The researchers are therefore relying on a priority access to Jean Zay and on the molecular dynamics simulation software Tinker-HP.
Deep learning for precision medicine
The aim of precision medicine, an emerging approach for the treatment and prevention of diseases that takes account of the patient’s genetic characteristics, environment and lifestyle, is t have a more personalised and predictive healthcare.
This medicine should enable the refinement of diagnoses and the development of treatments that are more efficient and adapted to individuals, as well as better detect the development of diseases and follow their progression.
At the heart of this precision medicine are the data produced by “omics” technologies and sciences, i.e. the set of tools that enable us to understand the operation of complex biological systems based on the analysis of massive data coming from several disciplines of molecular biology (genomics, metabolomics, proteomics, etc.).
Omics data is complex and heterogeneous, so researchers are now turning towards deep learning methods in order to extract knowledge from it.
These algorithms make it possible to analyse and combine omics data so as to discover, for example, new biomarkers, but also to combine them with other data such as electronic medical records, medical imaging, or the data from smart sensors.
Broadly speaking, deep learning is used more and more as a predictive and diagnostic assistance tool. It has yielded interesting results in the early detection of cancer.
Examples include a deep learning algorithm that can predict a risk of breast cancer before it actually develops, another that is capable of classifying melanoma with the precision of a dermatologist, and a third that can automatically detect colorectal polyps.
Blockchain for securing medical data
In order to fulfil their potential, these technologies require a huge amount of data (medical images, data from clinical trials, etc.), including patient data collected by doctors, hospitals or laboratories, whose security is a major issue for the healthcare sector.
Another issue is that of their interoperability: the exchange of information among the different healthcare stakeholders is often long and costly, with the various IT systems not having always been thought out to interact with one another.
In this context, blockchain appears as a means of guaranteeing the security and integrity of data – thanks to encryption and its distributed nature –, but also of solving interoperability problems by favouring the standardisation of data thanks to smart contracts for example.
In Estonia for example, the GuardTime company, in cooperation with the Estonian eHealth Foundation, has used blockchain to secure all the activity logs of the health records of the entire population, making it easy to detect any alterations or breaches of security.
In Europe, the MyHealthMyData project, carried by the European Commission, has the aim of designing a peer-to-peer architecture based on a private blockchain, to enable the exchange of data securely and in compliance with the General data Protection Regulation (GDPR).
For patients, this would translate as the creation of a personal account enabling them to access their digital health record, aggregate data from a variety of sources, and make this data available for medical research.
Via dynamic consent, they could define the conditions of use of all or part of their data, anonymised or not, depending on the intended uses. They could also “cut off access to their data” in accordance with the right to be forgotten. Here, blockchain thus offers a framework enabling the patient to control, and potentially monetise, the access to this health data.










